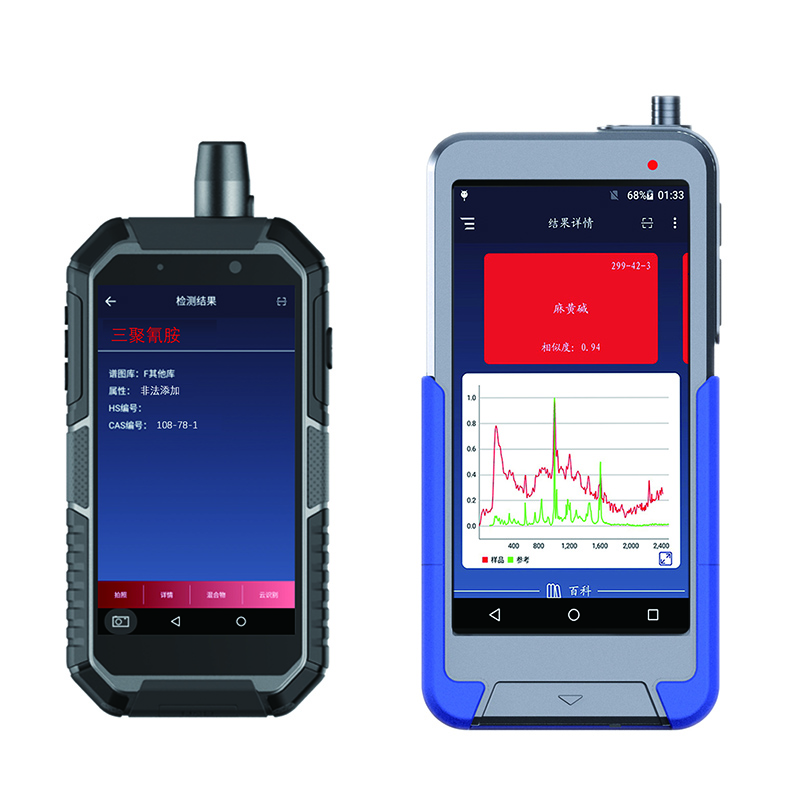
RS1000DI RS1500DI handheld Raman Identifier
★ Muaj ntau yam kev tshawb nrhiav, tshuaj, biochemical raw khoom, thiab pigments tuaj yeem txheeb xyuas
★ Nws tuaj yeem raug kuaj ncaj qha los ntawm iav, woven hnab, hnab ntawv, yas, thiab lwm yam ntim khoom (RS1500DI)
★ Me me thiab lub teeb yuag, nws tuaj yeem hloov tau yooj yim hauv warehouses, chav npaj khoom, kev cob qhia ntau lawm thiab lwm qhov chaw
★ Cov lus teb ceev thiab kev txheeb xyuas tuaj yeem ua tiav hauv vib nas this
★ Tsis tas yuav coj mus kuaj, tsis tas yuav hloov cov ntaub ntawv nyoos thiab cov khoom siv rau hauv chav kuaj, uas tuaj yeem zam tsis txhob kuaj cov kab mob.
★ Kev txheeb xyuas qhov tseeb, siv cov tshuab kev kawm qib siab, muaj zog tshwj xeeb
RS1000DI thiab RS1500DI
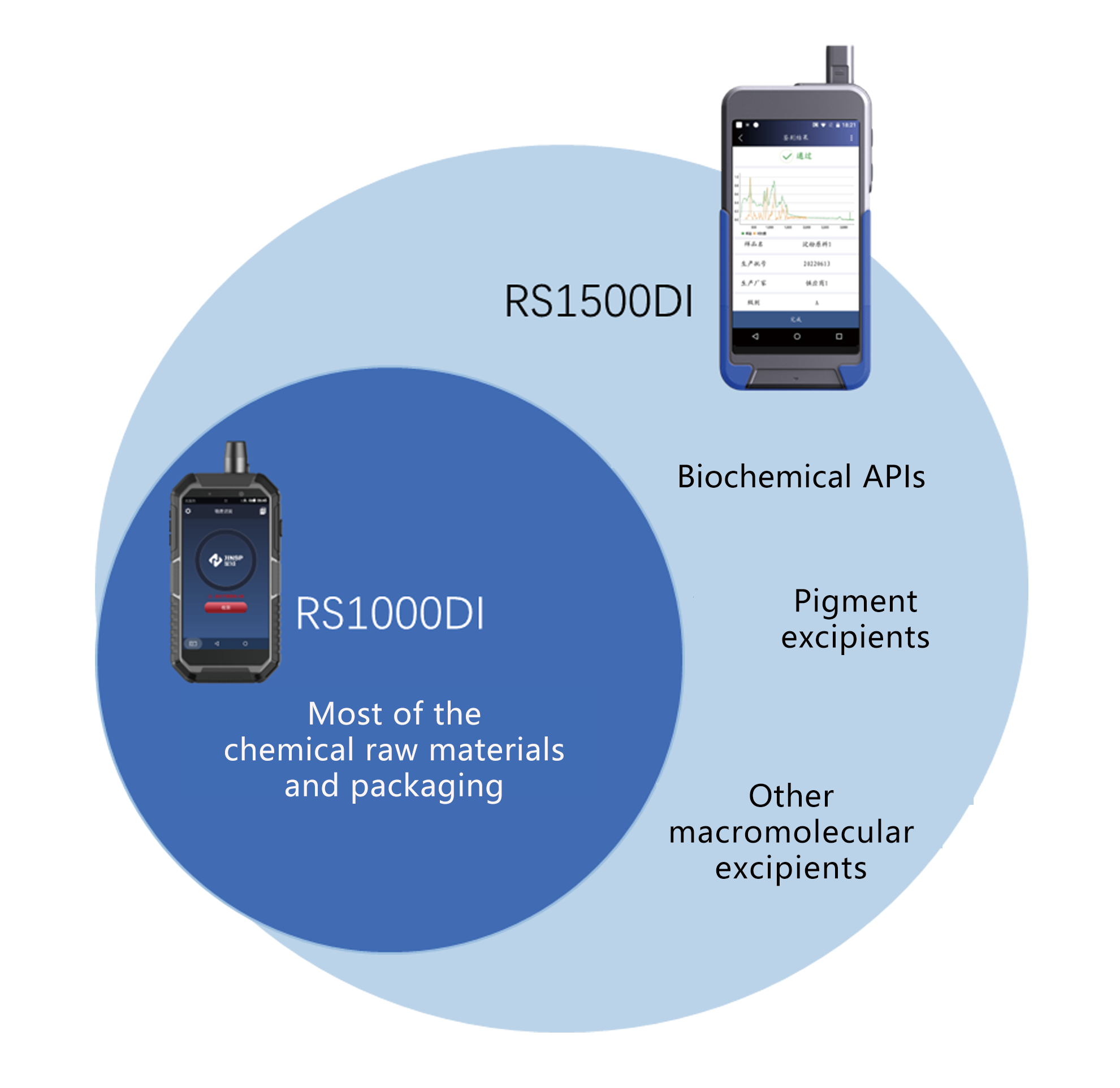
• Tshuaj raw khoom: tshuaj aspirin, acetaminophen, folic acid, niacinamide, thiab lwm yam.
• Pharmaceutical excipients: ntsev, alkalis, qab zib, esters, cawv, phenols, thiab lwm yam.
• Cov khoom ntim: polyethene, polypropylene, polycarbonate, ethylene-vinyl acetate copolymer
RS 1500 IB
• Biochemical APIs: amino acids thiab lawv cov derivatives, enzymes thiab coenzymes, cov proteins
• Pigment excipients: carmine, carotene, curcumin, chlorophyll, thiab lwm yam.
• Lwm yam macromolecular excipients: gelatin, microcrystalline cellulose, thiab lwm yam.
RS 1500 IB:
| Specification | Kev piav qhia |
| Technology | Raman Technology |
| Laser | 1064nm ua |
| Wyim | 730g (nrog rau roj teeb) |
| Ckev sib raug zoo | USB / Wi-Fi / 4G / Bluetooth |
| Ptus tswv | Rechargeable Li-ion roj teeb |
| Dua format | SPC / txt / JEPG / PDF |
RS 1000 IB:
| Specification | Kev piav qhia |
| Laser | 785nm ua |
| Qhov hnyav | 500g (nrog rau roj teeb) |
| Kev sib txuas | USB / Wi-Fi / 4G / Bluetooth |
| Hwj chim | Rechargeable Li-ion roj teeb |
| Cov ntaub ntawv hom | SPC / txt / JEPG / PDF |
1. International Pharmaceutical Inspection Cooperation Program (PIC/S) thiab nws cov GMP Cov Lus Qhia:
Daim ntawv ntxiv 8 Kev kuaj xyuas cov khoom siv raw thiab cov khoom ntim Cov ntaub ntawv pov thawj ntawm tag nrho cov khoom tuaj yeem raug lees paub tsuas yog tom qab kev kuaj xyuas tus kheej raug coj mus kuaj ntawm cov qauv hauv txhua lub thawv ntim.
2. US FDA txoj kev tsim khoom zoo tam sim no US FDA GMP:
FDA 21 CFR Part 11: Rau txhua yam ntawm cov tshuaj, tsawg kawg yog ib qho kev kuaj xyuas tus kheej yuav tsum tau ua;
FDA Inspector's Instruction Manual: Ua yam tsawg kawg ib qho kev kuaj xyuas tus kheej rau txhua pawg ntawm txhua cov khoom siv raw.